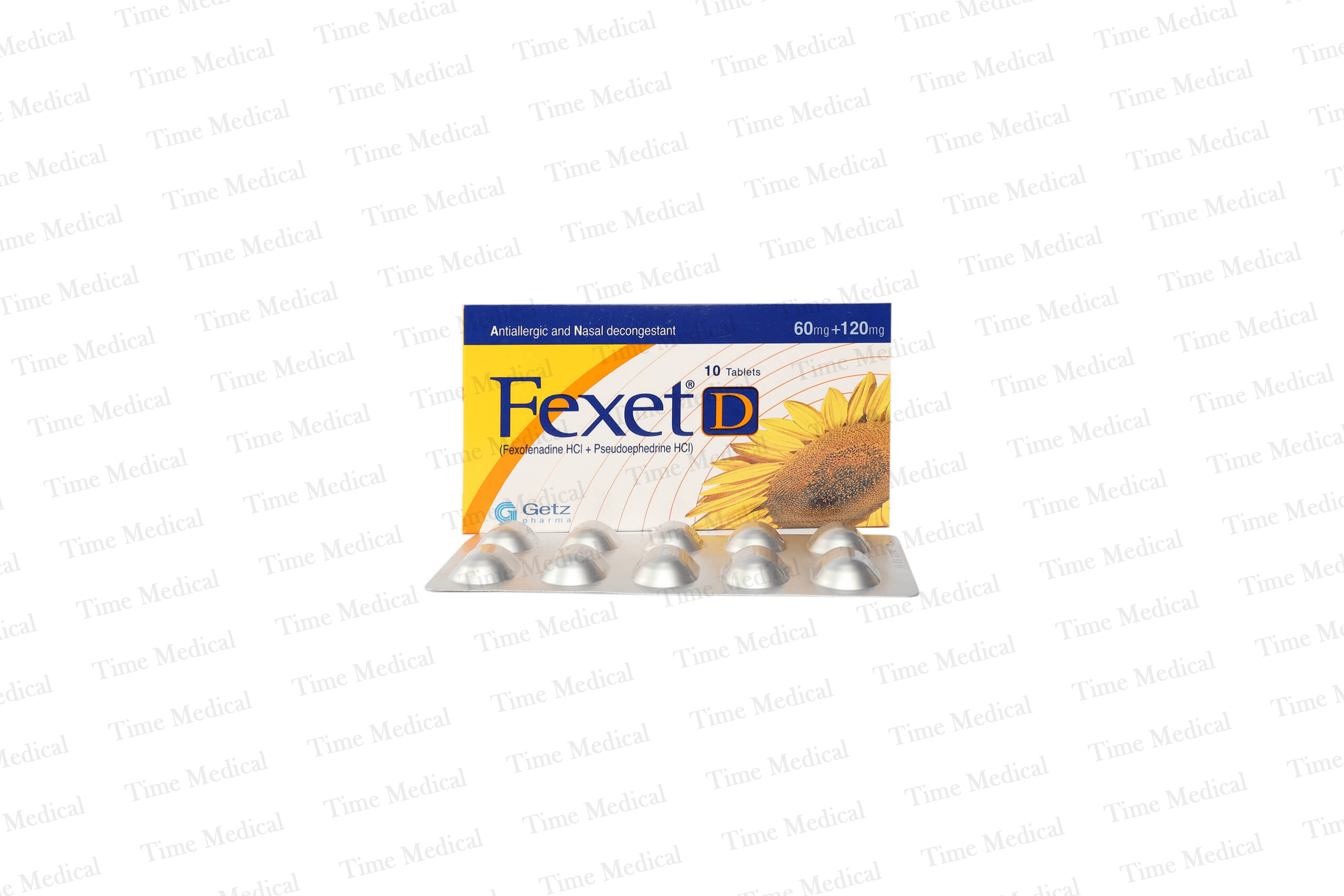
Description
Indications
Fexet-D Tablets 60/120mg is primarily indicated in conditions like Allergy, Chronic idiopathic urticaria, Cytotoxic-induced neutropenia, Hay fever, Rhinitis, Seasonal allergic rhinitis, Symptomatic relief of seasonal allergic rhinitis, Urticaria, and can also be given in adjunctive therapy as an alternative drug of choice in Runny nose, Sneezing.
Contraindication
Fexet-D Tablets 60/120mg
Side Effects
The severe or irreversible adverse effects of Fexet-D Tablets 60/120mg, which give rise to further complications include Viral infections. The signs and symptoms that are produced after the acute overdosage of Fexet-D Tablets 60/120mg include Convulsions, Coma, Hallucinations, Ataxia, Cardiorespiratory collapse, Tremor, Ventricular arrhythmias, Cardiovascular collapse, Sedation, Hyperreflexia, Psychosis, Excitement. The symptomatic adverse reactions produced by Fexet-D Tablets 60/120mg are more or less tolerable and if they become severe, they can be treated symptomatically, these include Dizziness, Headache, Drowsiness, Nausea, Vomiting, Diarrhea, Tinnitus, Hypotension, Hair loss, Hypersensitivity reactions, Blood dyscrasias, Increased intracranial pressure, Dysmenorrhea, CNS depression, Cold & flu, psychomotor impairment.
Warnings
Fexet-D Tablets 60/120mg should be used with caution in patients with kidney disease or any allergy, hepatic impairment. Do not take this medication for several days before any allergy testing since test results can be affected. Although drowsiness is uncommon at usual doses under normal circumstances, be aware that this medication may have such effects, before engaging in activities requiring alertness. This medication should be used only if clearly needed during pregnancy or lactation. Caution is advised when this drug is used in the elderly patients.




Reviews
There are no reviews yet.